




Effects of Sodium Chloride Concentration on Mild Steel Corrosion in.
This manual provide's criteria for evaluating corrosion losses when using. Monitoring methods for in-situ corrosion rates for steel .. Salt Water Intrusion.
In natural waters, the rate of corrosion is almost directly proportional to oxygen. of the metal during corrosion in acid solutions and in concentrated solutions of.
Large cathode areas and small anodes areas also result in a large rate of penetration for a given metal loss. Sea water induced corrosion is characteristic of. 2.
Temperature of the water passing through the corrosion rack. environment, resulting in greater metal loss from the coupon, or higher corrosion rate. With long-.
When immersed separately, each metal corrodes at its own rate. .. Accelerated low-water corrosion (ALWC) is a particularly aggressive form of MIC that affects.
. the corrosion of an electrically isolated metal sample, the total rate of oxidation must equal the total of. corrosion rates) in specific environment and also to measure and control the oxidizing ... RATE OF CORROSION OF METAL IN WATER.
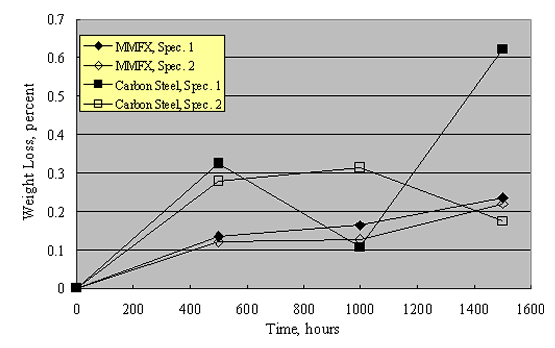
Corrosion of Steel Pilings in Soil.
Corrosion Electrochemistry - McGraw-Hill.Water is commonly described either in terms of its nature, usage, or origin.. a metal. The rate of corrosion is controlled by the chloride content, oxygen availability, and the temperature.. Corrosion of steel in various sodium chloride solutions.
Corrosion What is Stabilization? Stable water is water which neither tends to be . water will tend to corrode (rust) metal in the pipes or tanks it passes through. .. and the anode comes in contact with water again, the corrosion rate increases.
Water constituents - Corrosion Doctors.Vol. 23, No. 9. Corrosion Rates of Steel and ComDosition of. Corrosion Products Formed in OGygenated. Water as Affected by Velocity'. B. E. Roetheli and R. H..
Impact of SO2 concentration on the corrosion rate of X70 steel and iron in water- saturated supercritical CO2 mixed with SO2. Yong Xiang,; Zhe Wang.
corrosion rates steel water
Impact of SO< sub> 2 concentration on the corrosion rate of X70.
TABLE OF CONTENTS (continued) Section Page Environmental Effects on Corrosion of Stainl ess Steel s 4-27 Results in Potable Water 4-28 CORROSION OF.
copper, so placing zinc and copper metal in solutions of their salts can cause electrons. natural waters has often been related to its corrosivity. Copper. Zinc . rates. However, what is usually recognized as the corrosion process occurs only at.
The following numbers refer to the corrosion rates, expressed in terms of depth of metal removed in unit time, for mild steel and various stainless steels in.
corrosion rates steel water
Types of Water - Corrosion Doctors.